
Steve Fisher (Senior Author and Principal Investigator; Retinal Cell Biology Laboratory)
We have a new publication in a collaboration with Drs. Qirui Hu, Sheldon Miller, Peter Munson, Arvydas Maminiskis, and Bo Chang that examined the anatomical and genetic changes in the retina and underlying pigmented epithelium in a model of retinal detachment. Retinal detachment initiates a cascade of changes at the cellular and genetic level, understanding these changes allow for the development of therapeutic agents aimed at arresting the neurodegenerative process. That article in its entirety can be found here.
Abstract:
PURPOSE: The purpose of this study was to examine the rpea1 mouse whose retina spontaneously detaches from the underlying RPE as a potential model for studying the cellular effects of serous retinal detachment (SRD).
METHODS: Optical coherence tomography (OCT) was performed immediately prior to euthanasia; retinal tissue was subsequently prepared for Western blotting, microarray analysis, immunocytochemistry, and light and electron microscopy (LM, EM).
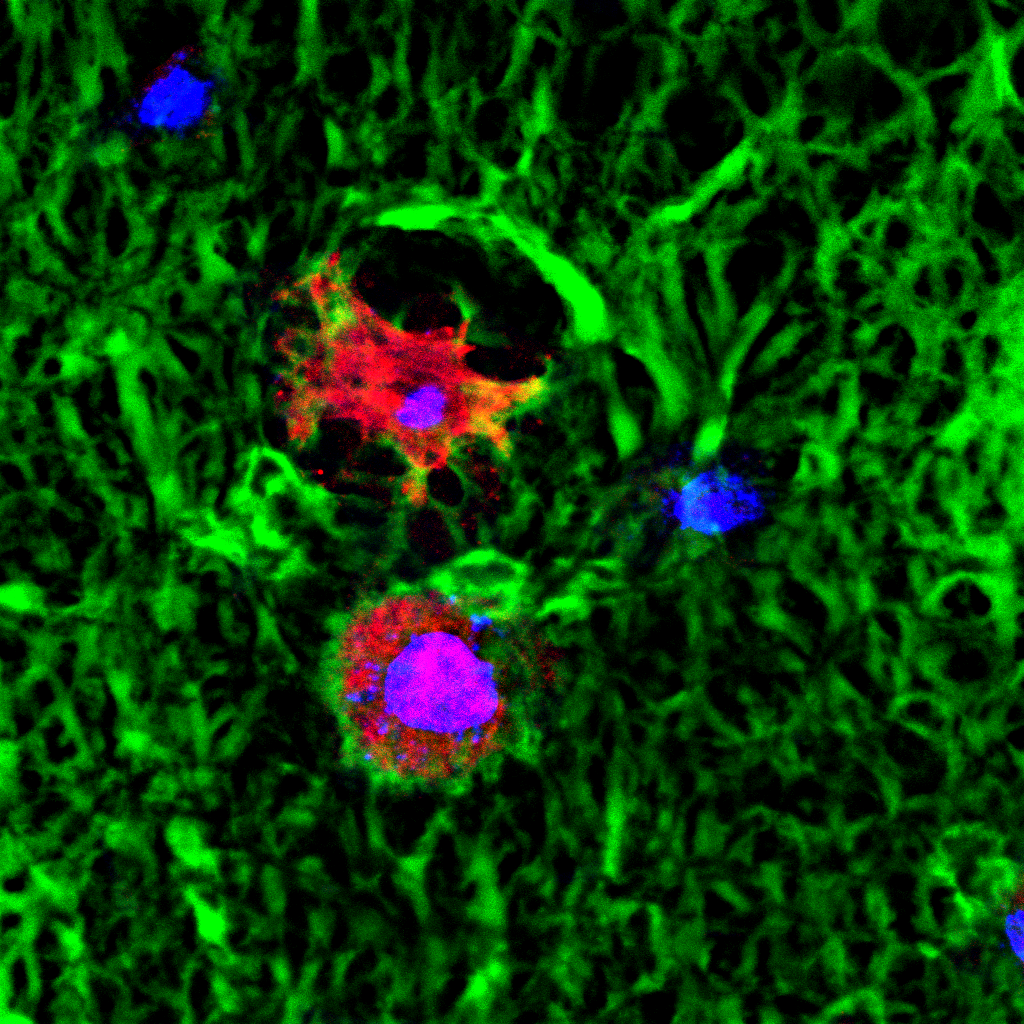
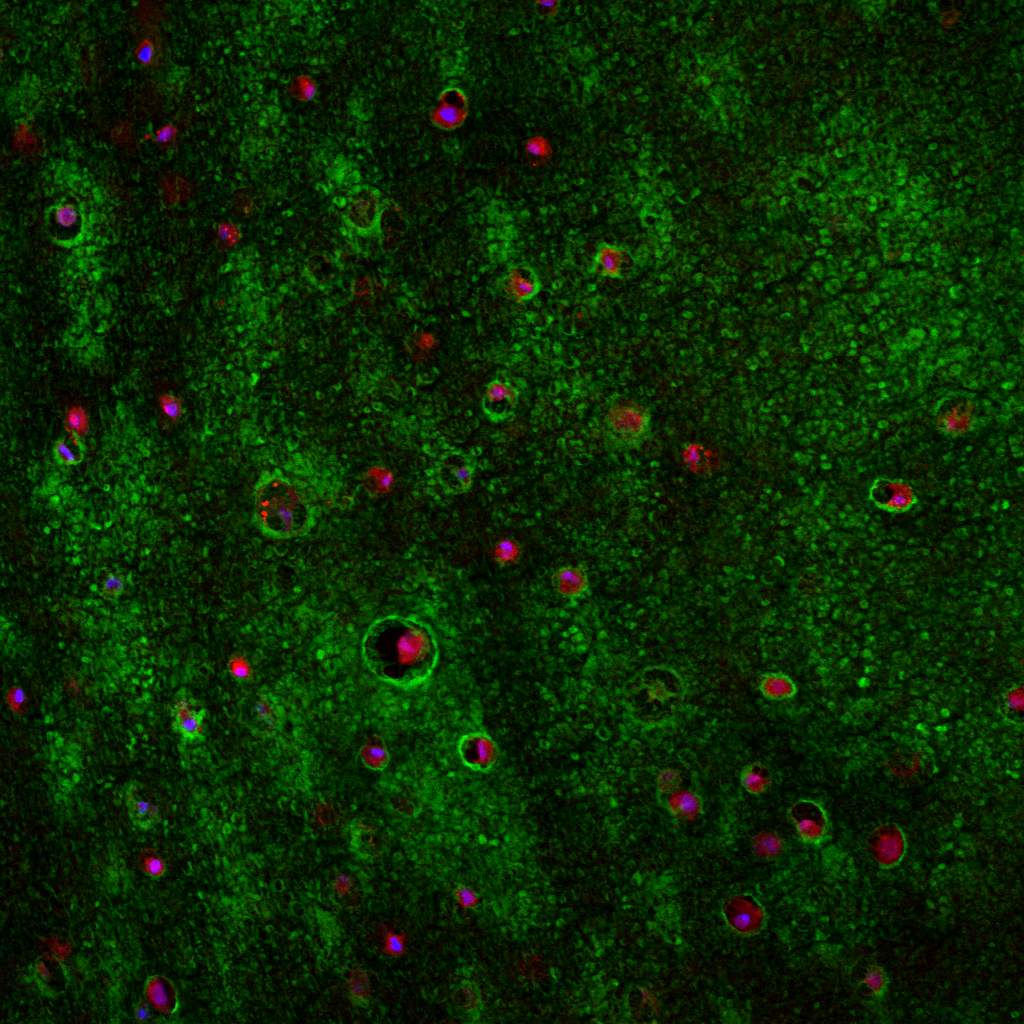
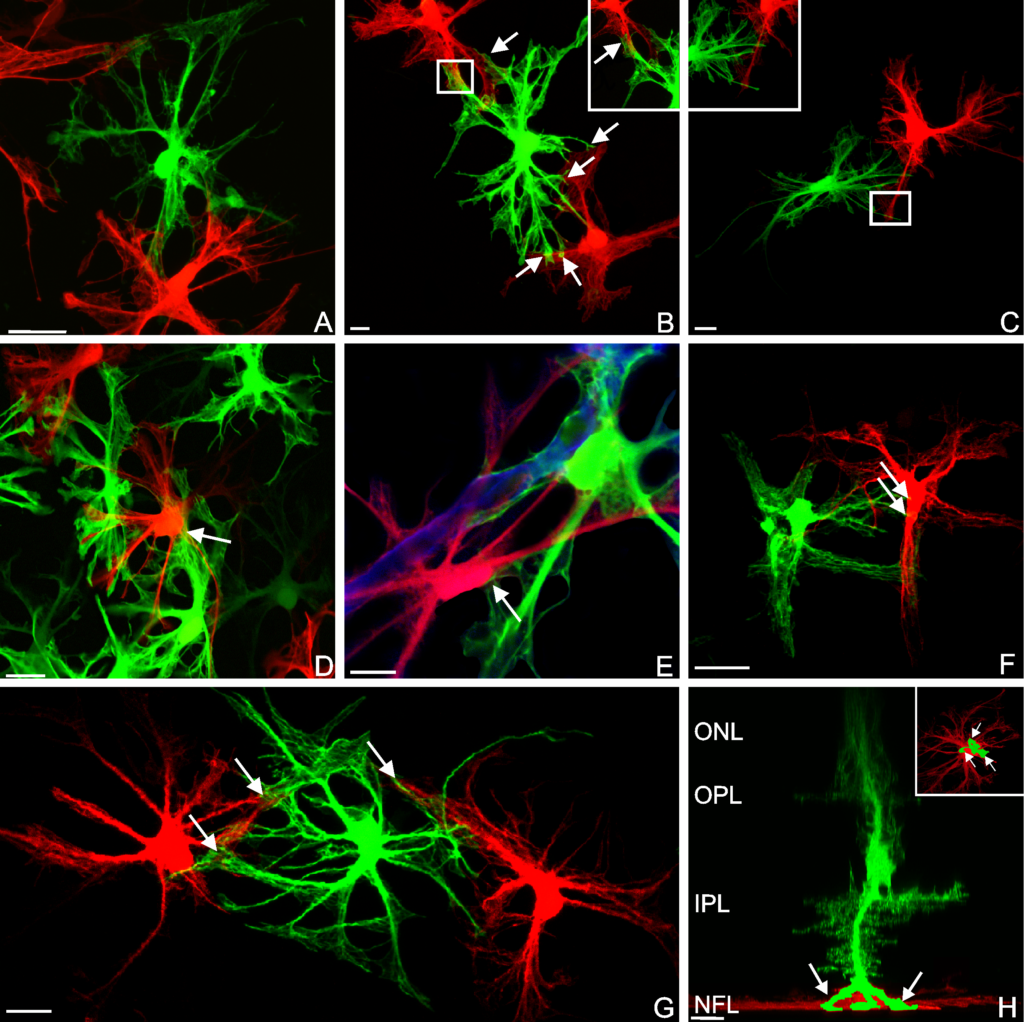
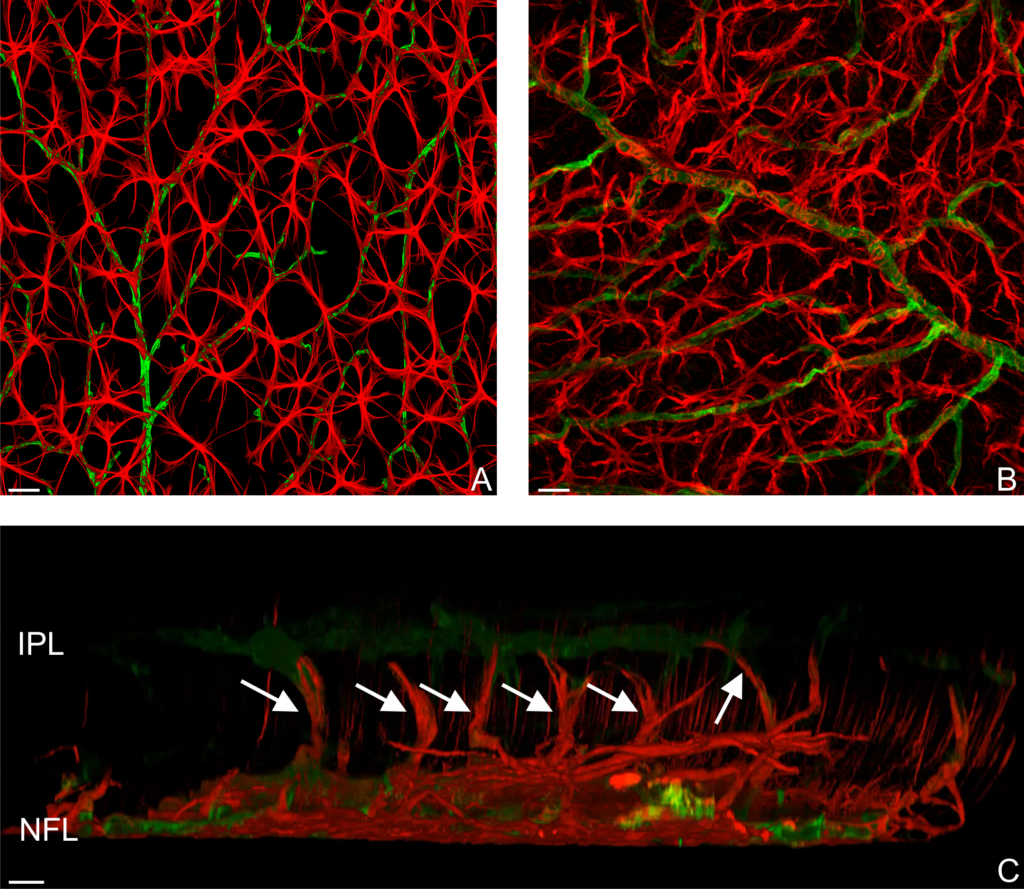
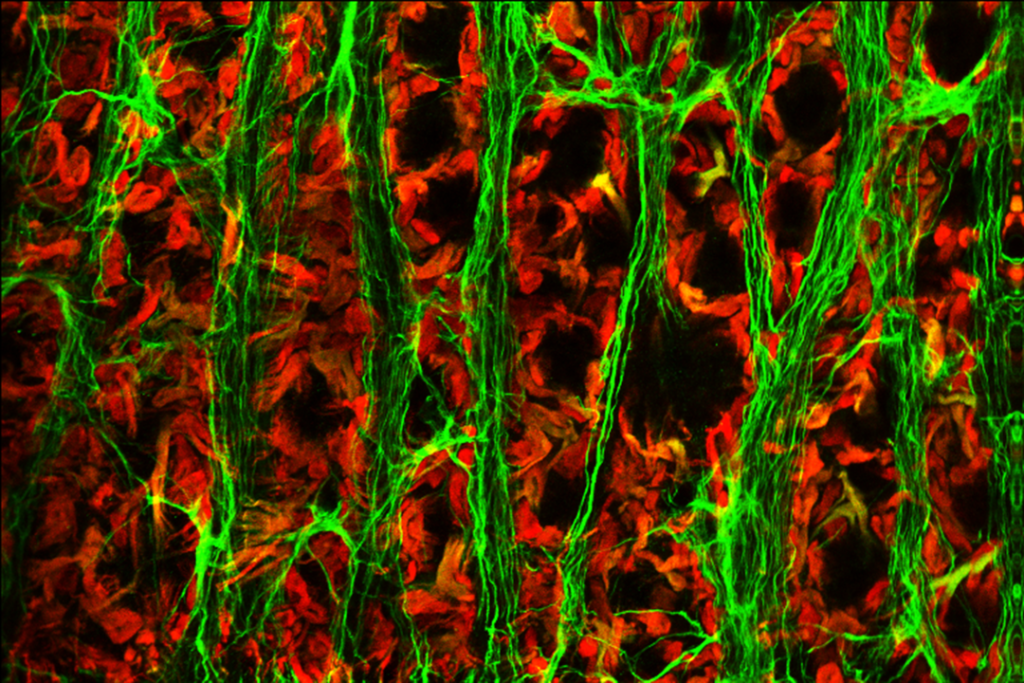
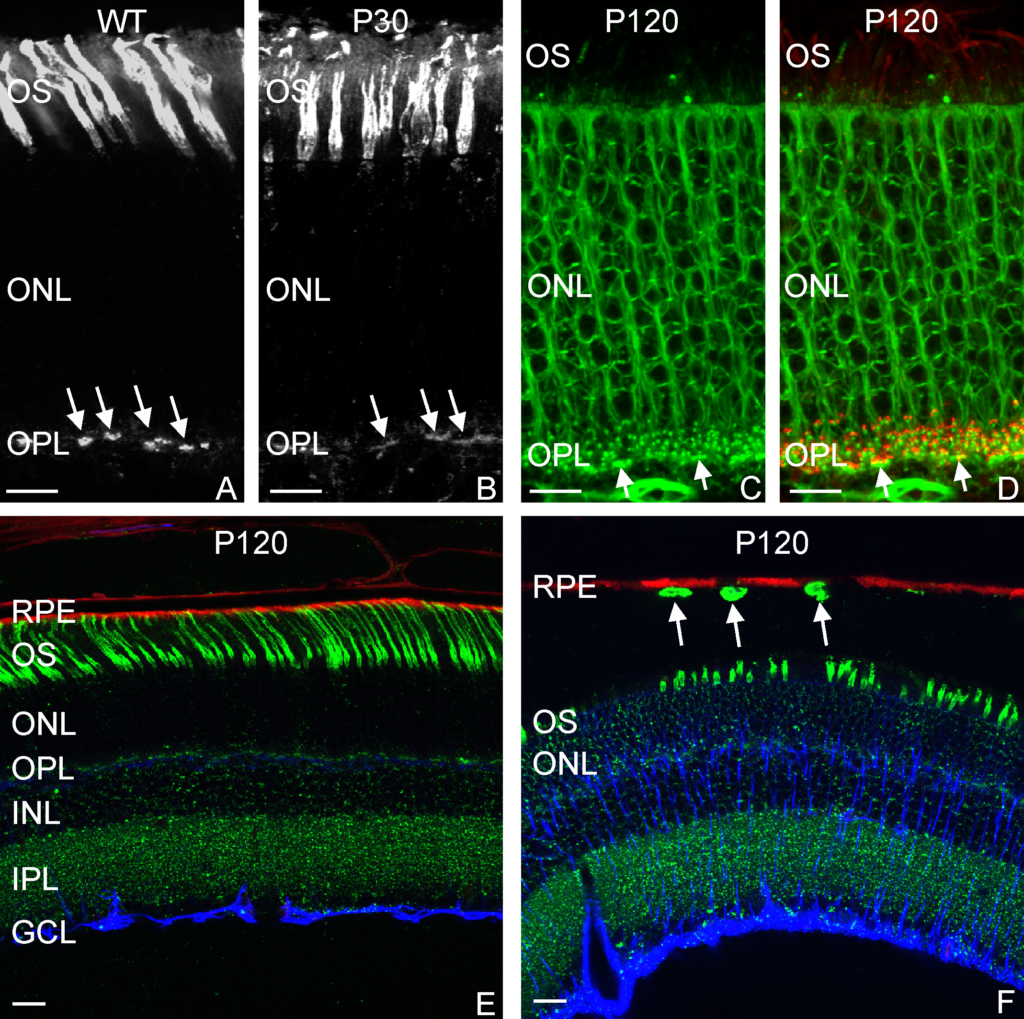
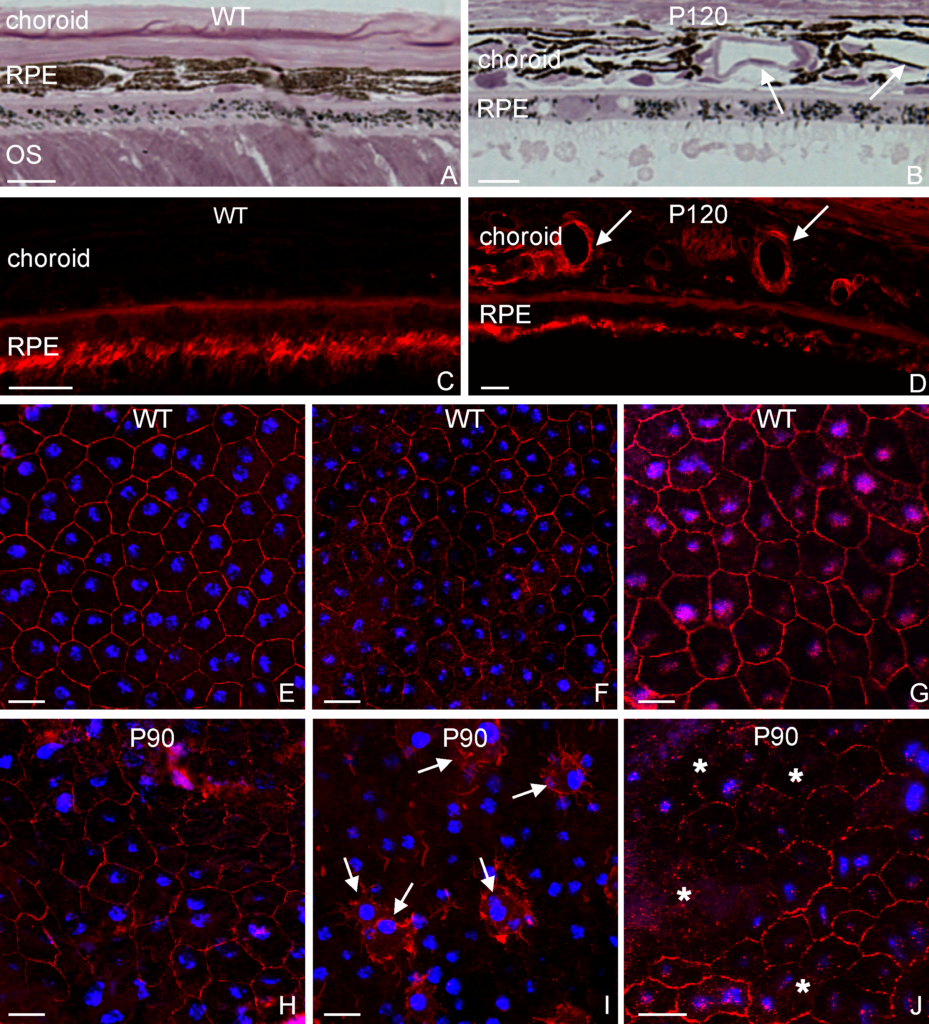
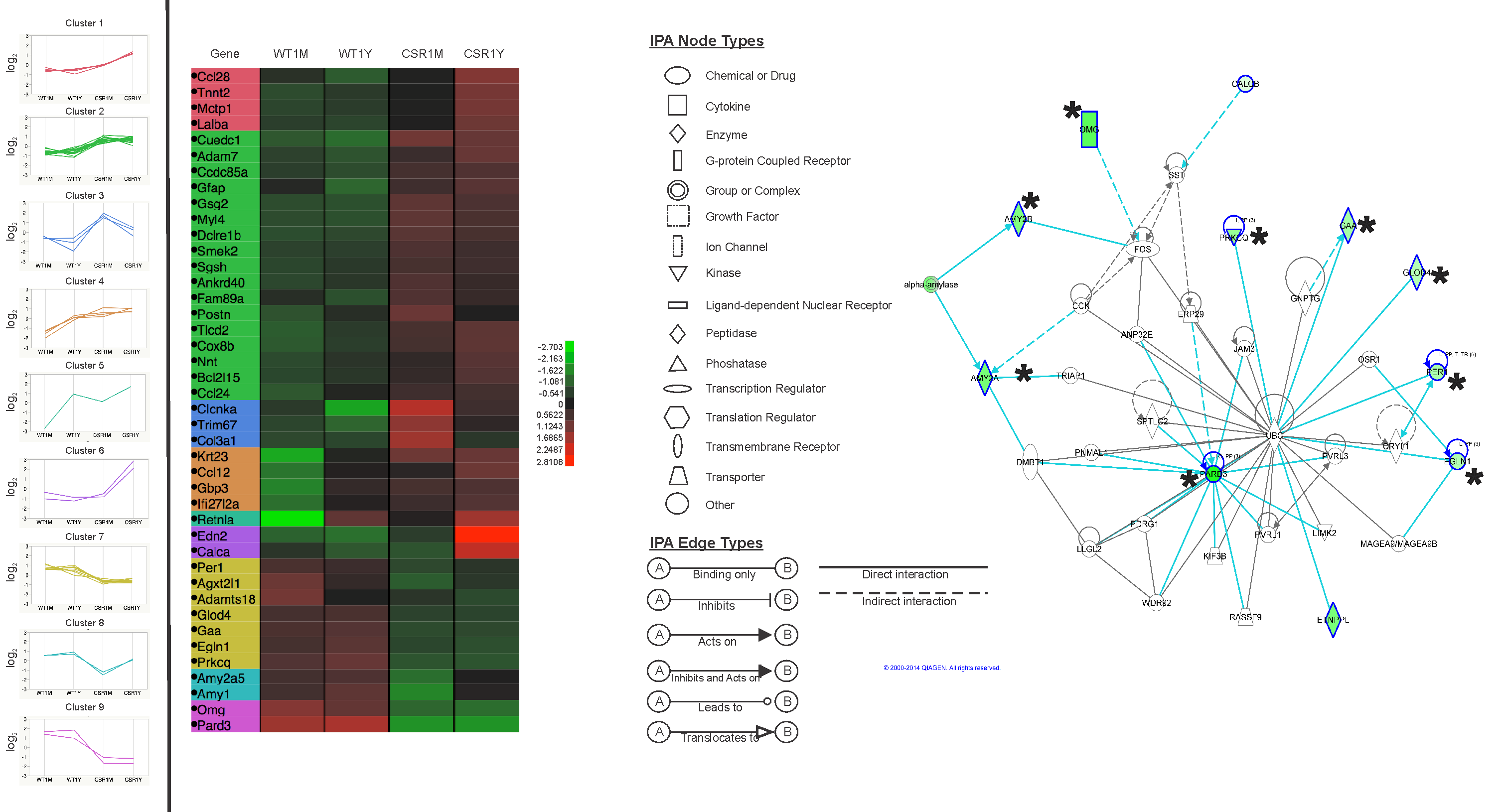
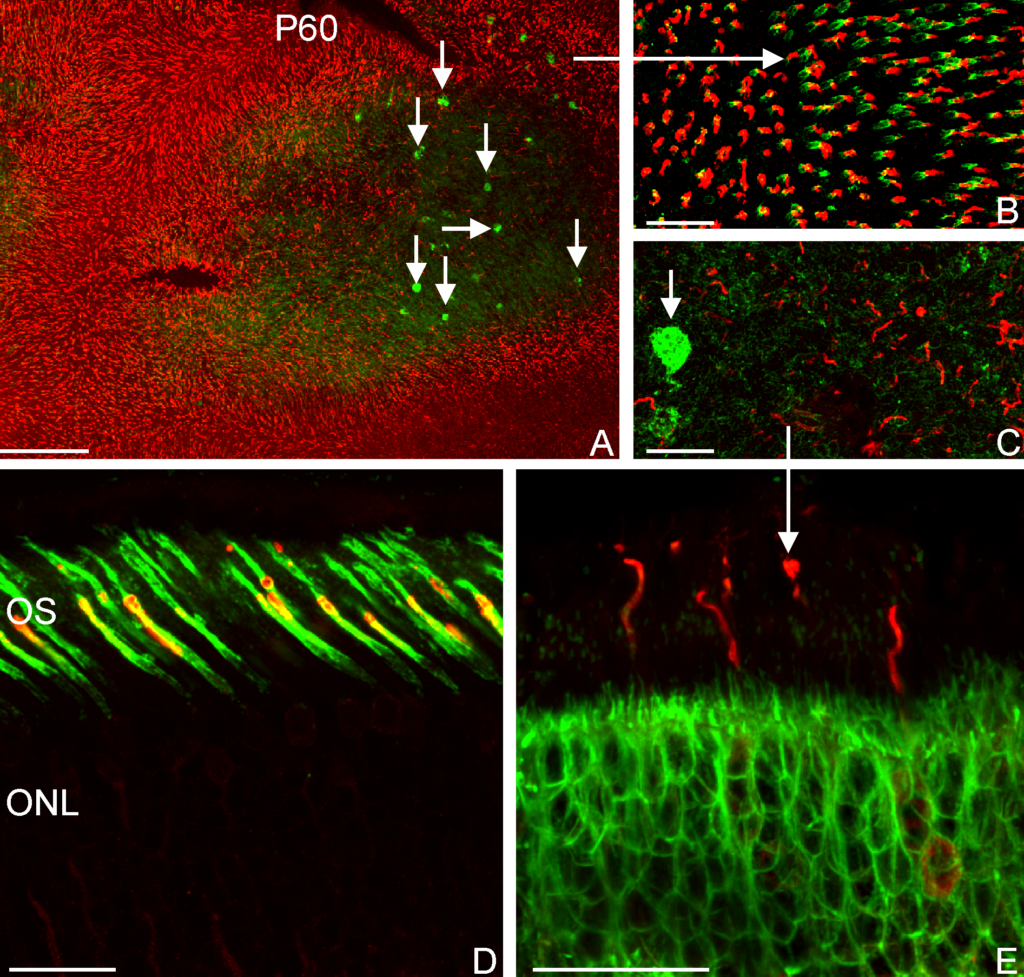
RESULTS: By postnatal day (P) 30, OCT, LM, and EM revealed the presence of small shallow detachments that increased in number and size over time. By P60 in regions of detachment, there was a dramatic loss of PNA binding around cones in the interphotoreceptor matrix and a concomitant increase in labeling of the outer nuclear layer and rod synaptic terminals. Retinal pigment epithelium wholemounts revealed a patchy loss in immunolabeling for both ezrin and aquaporin 1. Anti-ezrin labeling was lost from small regions of the RPE apical surface underlying detachments at P30. Labeling for tight-junction proteins provided a regular array of profiles outlining the periphery of RPE cells in wild-type tissue, however, this pattern was disrupted in the mutant as early as P30. Microarray analysis revealed a broad range of changes in genes involved in metabolism, signaling, cell polarity, and tight-junction organization.
CONCLUSIONS: These data indicate changes in this mutant mouse that may provide clues to the underlying mechanisms of SRD in humans. Importantly, these changes include the production of multiple spontaneous detachments without the presence of a retinal tear or significant degeneration of outer segments, changes in the expression of proteins involved in adhesion and fluid transport, and a disrupted organization of RPE tight junctions that may contribute to the formation of focal detachments.